COVID-19 and Viscoelastic Hemostasis Assays: Frequently Asked Questions
Please review ASH's disclaimer regarding the use of the following information. The FAQs available on this page are not being regularly updated. The information contained herein is only accurate as of the date listed, which represents the last time the information was reviewed by experts. For the latest information on COVID-19 treatments, please review ASH’s COVID resources; previously available resources can be accessed via the archives.
(Version 2.0; last updated February 25, 2021)
Input from Oksana Volod, MD; Rita Selby, MBBS, FRCPC; Morayma Reyes Gil, MD, PhD; Lisa Baumann Kreuziger, MD; and Agnes Y. Y. Lee, MD, MSc.
Note: Please review ASH's disclaimer regarding the use of the following information.
What are Viscoelastic (VE) assays and what do they measure?
Viscoelasticity refers to the property of materials that exhibit both viscous and elastic characteristics when undergoing deformation. Blood, which is a viscous material undergoes significant changes during coagulation and becomes less viscous and more elastic in nature. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are both VE global tests of coagulation performed on whole blood. They assess clot formation, clot strength, and dissolution by measuring the amount of a continuously applied rotational force transmitted to an electromechanical transduction (TEG) or optical detection (ROTEM) system, with results displayed as a graph. While TEG® Hemostasis Analyzer System (Haemonetics Corp.) and ROTEM® delta (Instrumentation Laboratory) have been the principal VE technologies in use so far, other VE testing platforms have recently been approved by the U.S. Food and Drug Administration (FDA) and introduced for clinical use in the United States, including the TEG®6s system (Haemonetics Corp.) and the Quantra® Hemastasis Analyzer (HemoSonics LLC). Both the TEG 6s and Quantra systems are cartridge-based with dry reagents and fully automated, designed for improved usability while testing at point-of-care. Several reviews on VE technologies have been published recently.1,2 Regardless of principle or method of detection, all VE technologies assess rate of clot formation, kinetics, strength, and lysis, as seen in superimposed representative graphs for TEG and ROTEM (Figure). The Table summarizes descriptions of the various VE assay parameters and their clinical significance.
What is the impact of anticoagulation or antiplatelet agents on VE assays?
Kaolin or other intrinsic/contact phase activators are used to initiate standard VE assays. Therefore, VE assays are more sensitive to agents such as unfractionated heparin (UFH), low-molecular-weight heparin, and direct thrombin inhibitors. Tissue factor (extrinsic phase activator) is used in some VE assays (e.g., TEG CRT and ROTEM EXTEM) but is less sensitive to warfarin and direct Xa inhibitors. VE assays can distinguish heparin effect via addition of heparinase to the assay; however, these assays have not been validated to monitor anticoagulation. VE assays were not originally designed to measure inhibition of specific platelet receptor activities resulting from antiplatelet therapies (e.g., clopidogrel and aspirin), since TEG maximal amplitude, ROTEM maximal clot firmness (MCF), and Quantra clot stiffness (CS) assess platelet function following maximal thrombin activation via the protease-activated receptor 1 (PAR1), which is unaffected by antiplatelet agents. A modified TEG and TEG 6s assay, Platelet MappingTM (TPM), allows for the assessment of non–thrombin-based platelet activation/inhibition.
How are VE tests being used in COVID-19 patients?
Conventional coagulation tests (CCT) do not reflect the complexity of hemostatic alterations and are not available for immediate decision-making at point of care. Because seriously ill COVID-19 patients may have marked abnormalities in hemostasis and thrombosis, clinicians managing severe COVID-19 are interested in using tests that may help them quickly understand whether an individual patient is hypocoagulable, hypercoagulable, or has abnormal fibrinolysis. Most published reports are case reports/series or small prospective or retrospective observational studies, but what we are learning from these studies is that unlike the “typical” sepsis-induced disseminated intravascular coagulation (DIC), COVID-19–associated coagulopathy is characterized by a progression to a more pronounced hypercoagulable state in advanced disease rather than the hypocoagulability observed in late sepsis-induced DIC, at least in most cases.3 COVID-19 hypercoagulability measured by VE assays reveals not only an increased rate of thrombin generation, but also increased contributions of fibrin and platelets to clot stability and stiffness (high TEG maximal amplitude, ROTEM MCF, or Quantra platelet contribution to stiffness, and fibrinogen contribution to stiffness parameters). The FDA has recently issued guidance regarding the expansion of whole blood VE measurements to COVID-19 patients, beyond standard clinical settings like cardiac surgery and trauma, because currently no devices have been approved for use. The FDA has indicated that use of these devices in hospitals may provide real-time assessment of whole-blood VE properties and aid in patient management.
Can VE assays be used to detect hypercoagulability or optimize thrombosis prevention in COVID-19 patients?
Currently, both National Institutes of Health (NIH) and ASH guidelines recommend administration of prophylactic dosing of anticoagulation in the setting of critically ill or hospitalized patients in the absence of documented thromboses and outside of clinical trials. Intensification of anticoagulant therapy or use of antiplatelet agents based on platelet count, D-dimer levels, or other conventional coagulation markers is currently not recommended. Several single-center, prospective, and retrospective observational studies published recently have used TEG, ROTEM, and Quantra devices to assess hospitalized or critically ill COVID-19 patients, and some, but not all, have reported that VE testing (VET) hypercoagulability parameters predicted higher rates of thrombosis in COVID-19 patients.4-8 One single-center observational study demonstrated improved critical care outcomes when an institution-specific TEG-Platelet Mapping guided thromboprophylaxis regimen incorporating antiplatelet agents was used compared to standard of care.9 However, patients were not randomized between VET- and non-VET–based care, and significant differences in baseline characteristics may have also influenced outcomes.
Although all VE devices measure the VE properties of whole blood, VE parameters are not interchangeable across devices. Therefore, device-specific algorithms must be developed for COVID-19 applications. The FDA VE test-related guidelines may help clinicians incorporate VE devices into algorithms to stratify COVID-19 patients into hypercoagulable, hypercoagulable, hypofibrinolytic, or hyperfibrinolytic phenotypes, and advance further study of the utility and clinical outcomes associated with VE test associated management algorithms in larger multicenter randomized controlled trials.
References:
- Carll T, Wool GD. Basic principles of viscoelastic testing. Transfusion. 2020;60:S1–S9.
- Selby R. “TEG Talk”: Expanding Clinical Roles for Thromboelastography and Rotational Thromboelastometry.” Hematology Am Soc Hematol Educ Program 2020 Dec 4;2020(1)67-75.
- Gergi M, Goodwin A, Freeman K, et al. Viscoelastic hemostasis assays in septic, critically ill coronavirus disease 2019 patients: a practical guide for clinicians. Blood Coagul Fibrinolysis. 2021 Jan 12. doi: 10.1097/MBC.0000000000000999. Epub ahead of print. PMID: 33443923.
- Mortus JR, Manek SE, Brubaker LS, et al. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open. 2020;3:e2011192.
- Almskog LM, Wikman A, Svensson J, et al. Rotational thromboelastometry results are associated with care level in COVID-19 [published online ahead of print, 2020 Oct 17]. J Thromb Thrombolysis. 2020;1-9.
- Ranucci, M., Ballotta, A., Di Dedda, U, et al., The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. J Thromb Haemost, 2020; 18: 1747-1751. https://doi.org/10.1111/jth.14854.
- Masi P, Hékimian G, Lejeune M, et al., Systemic Inflammatory Response Syndrome Is a Major Contributor to COVID-19–Associated Coagulopathy: Insights From a Prospective, Single-Center Cohort Study. Circulation. 2020 Aug 11;142(6):611-4.
- van Veenendaal N, Scheeren TWL, Meijer K, et al. Rotational thromboelastometry to assess hypercoagulability in COVID-19 patients. Thromb Res. 2020;196:379-381.
- Hranjec T, Estreicher M, Rogers B, et al. Integral use of thromboelastography with platelet mapping to guide appropriate treatment, avoid complications, and improve survival of patients with coronavirus disease 2019-related coagulopathy. Crit Care Explor. 2020;2(12):e0287.
For additional information, see:
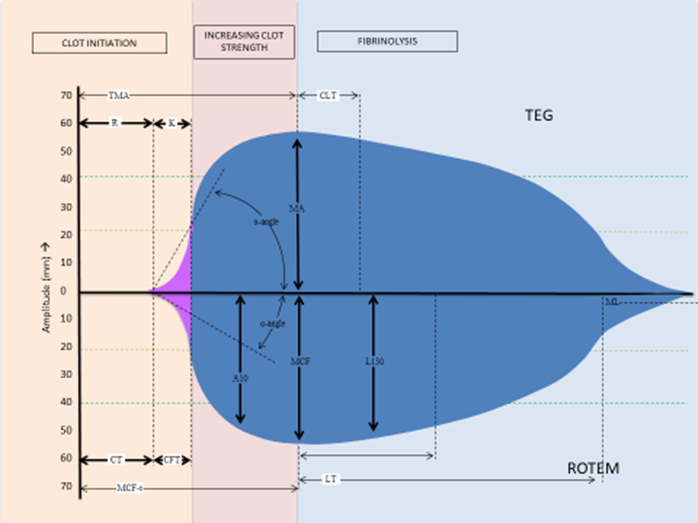
Figure. Superimposed representative graphs and parameters for TEG and ROTEM. TEG parameters: R, reaction time; K, kinetics; α,-angle; MA, maximum amplitude; CLT, clot lysis time (time taken for amplitude to decrease by 2 mm from MA). ROTEM parameters: CT, clotting time; CFT, clot formation time; α,-angle; A10, amplitude at 10 min; MCF-t, time to maximum clot firmness; MCF, maximum clot firmness; LOT, lysis onset time; LT, lysis time (time taken from amplitude to drop by 10% of MCF); LI30, lysis Index at 30 min (% drop in amplitude from MCF); ML, maximum lysis (minimum amplitude achieved at the end of test run time).
Table. Comparison of Viscoelastic Test Parameters
| Viscoelastic Assay | Main Contributor | TEG® 5000 | TEG 6s Global Cartridge | ROTEM® Delta or Sigma | Quantra® QPlus® System | Clinical Significance |
| Clot initiation | Coagulation factors |
R, minutes Reaction time |
CK R, minutes Citrated kaolin R |
CT, seconds Clotting time |
CT (Clot Time), seconds CTH (Heparinase Clot Time), seconds CTR (Clot Time Ratio) |
A short (R or CT or CTH) time indicates a hypercoagulable state and a prolonged (R or CT) time indicates either hypocoagulability or the presence of an anticoagulant. A short CTH in the presence of a long CT indicates the presence of heparin anticoagulation. |
| Clot kinetics | Fibrinogen |
K, minutes Kinetic time |
CFF MA Citrated functional fibrinogen |
CFT, seconds Clot formation time |
FCS (Fibrinogen Contribution to stiffness), hPa |
Angle reflects fibrin kinetics, including fibrin formation and cross-linking. FCS measures the direct contribution of stiffness generated by fibrinogen. |
| Clot stiffness | Fibrinogen, Platelets |
MA, mm Maximum amplitude |
CK MA, mm CRT MA, mm |
A10, A20, mm Amplitude 10, 20 min after CT |
CS (Clot Stiffness), hPA * Unique to Quantra |
MA, MCF, and CS reflect platelet and fibrinogen contributions to the clot stiffness and full platelet potential under maximal stimulation by thrombin. PCS isolates the platelet contribution to clot stiffness. |
| Clot stability | Fibrinolytic enzymes, inhibitors, Factor XIII |
LY30, % Lysis 30 minutes after MA is reached |
NA | LI 30, LI 60 % of MCF Lysis index at 30 and 60 minutes, after CT |
NA |
In VE assays, normal fibrin clots are stable for a few hours. Evidence of clot lysis that starts within 30 minutes of clot formation suggests hyperfibrinolysis. |