ASH Agenda for Hematology Research
The ASH Agenda for Hematology Research serves as a roadmap to prioritize research within the hematology field. In it, we identify key emerging and transformative areas of research that will launch the field into the next generation of therapies for hematologic conditions. The Agenda is updated periodically and is designed to be a living document. ASH encourages everyone in the hematology community to embrace the ASH Agenda for Hematology Research as a resource and consider citing it in publications, grant applications, or other efforts.
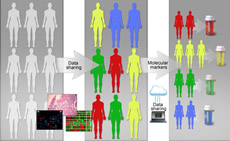
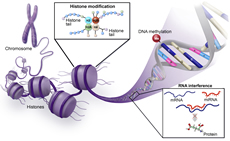
Genetics, Genomics, and Epigenetics
Gene discovery science has been a rich source of innovation in hematology, and hematology has been an ideal testing ground for technologic innovation in this area. Hematology has advanced genetics, genomics, and epigenetics through increased application of technologies in precision medicine, including mutational analyses, epigenetic modulation and gene control, and transcriptomics. Continued development and use of new analytic platforms including single cell sequencing, epigenomics, and proteomics will further broaden this area and its impact on hematology and patient outcomes.
Immunology and Immunotherapies
Understanding of basic immunology is essential to unlock the underpinnings of hematologic disease. Likewise, immunotherapy has revolutionized treatment for patients and is rapidly being applied to non-malignant disease to improve outcomes. Emphasis on both basic immunology and applications of immunotherapy is central to improved care in hematologic diseases as fundamentals of immunology, including B/T cell and thymus function, has broad applications to hematopoietic stem cell transplantation, transfusion medicine, cellular therapy, and novel immune based therapies.
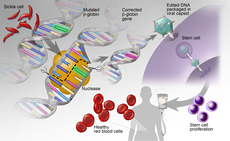
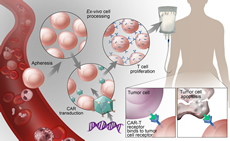
Gene Editing and Cellular Therapy
Advances in gene editing and cellular therapies offer promising new therapeutic options that are poised to revolutionize care for those with hematologic conditions. We are at the forefront of understanding how to integrate genomic data with technologies to alter gene expression and cellular function to combat and eliminate disease.
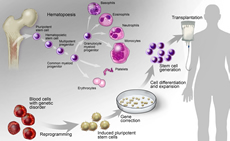
Development of Hematopoietic Progenitor and Stem Cells
Our fundamental understanding of stem cell function in hematopoiesis is rapidly changing due to improved tools and new insights, including single cell “omics” technologies and high-resolution single cell and in situ imaging. Research in this area will fuel mechanistic discoveries and translational breakthroughs.
Infectious Diseases and Hematology
The established relationship between host defense and blood includes complex and pleiotropic roles for both hematopoietic cells and coagulation factors during infection and subsequent immunity. Newly described functions for neutrophils, platelets, red blood cells, and soluble proteins as immunoregulators in both viral and bacterial pathogens suggest evolutionarily conserved interactions representing novel molecular targets that can be exploited for prevention or treatment. The need for knowledge in this area is underscored by global health emergencies including the emergence of coronaviruses, including SARS-CoV-2, which have exposed critical gaps in our understanding of blood and blood vessels.
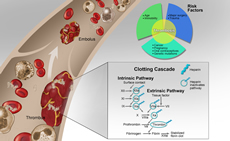
Thrombosis and Vascular Biology
In spite of significant advances in understanding the interplay between vessels, blood, and hemodynamics in clotting, essential questions about thrombosis and thromboembolic disease persist. The emergence of COVID-19 infection and its association with thromboembolic risk underscores gaps in our understanding of the role of vasculopathy and endothelial dysfunction in hematologic disease.
Cross-cutting Themes
Looking to the future, these areas represent some of the most promising strategies to overcome the limitations of current therapies and accelerate momentum to cure hematologic diseases. Dedicated research funds will enable the pursuit of these compelling opportunities and will equip the next generation of scientists in the field to produce high-impact results and ultimately introduce new standards of care that will not only transform the diagnosis and treatment of patients with hematologic diseases today, but will form the basis for continued scientific progress in hematology and other fields of medicine for years to come.
Development, control, regulation, and evolution of hematologic function and disorders occur across the age continuum from newborns to the elderly. It is critical to investigate age-related changes in the hematopoietic system and implications for development, diagnosis, progression, and treatment of non-malignant and malignant hematologic diseases.
We are entering an era where analysis and use of large datasets will have an increasing impact on disease prevention, detection, and treatment. Increased understanding of the structure and interplay of the genome, epigenome, transcriptome, proteome, microbiome, metabolome and 3-dimensional cellular and tissue structure at high resolution and often single cell level, and the development of sophisticated analytic approaches to decipher high content data, is imperative to define biologic mechanisms and inform clinical translation.
Links between basic/discovery science and the clinic have never been stronger. Clinical observations inform studies that uncover novel mechanisms of disease pathogenesis, and there are increasing examples of how basic/discovery science provides new ideas that are directly translated into clinical treatments that improve patient outcomes. Strengthening the interplay between basic/discovery science and clinical applications will increase opportunities to link clinical research with mechanistic insights. Expansion of population-scale databases with clinical and biomarker data will empower and guide discovery science and ensure it is patient-focused.
Disparities in health outcomes for racial, ethnic, gender, and other underrepresented populations have been an unfortunate and unacceptable part of research and healthcare for centuries. Studies aimed at understanding the impact of health disparities on malignant and non-malignant hematologic disorders, and the developing strategies to reverse such disparities in hematology are of central importance. We must understand the drivers of disparities and how they relate to patient outcomes in malignant and non-malignant disorders. Central to this are (a) the design of clinical trials and enrollment processes that are more inclusive, represent the population of those affected by the disease, and likely to benefit from the treatment and (b) studies into the basis of health disparities and their impact on hematologic disorders and (C) increased efforts to include diversity and health disparities in preclinical discovery and translational science.
Investigators driving research programs, particularly at the leadership level, have also lacked diversity. We must recruit and train a diverse workforce that includes under-represented racial and ethnic groups, women, and others who have been marginalized within the profession. Likewise, access to and support for careers in research and patient care must be improved so all can access and benefit from the scientific advances.
Acknowledgements
The Agenda for Hematology Research is developed by ASH’s Committee on Scientific Affairs with extensive input from the Society’s 18 scientific committees, ASH Executive Committee and individual members. ASH thanks all members who contributed to this effort.
Questions?
For more information about the ASH Agenda for Hematology Research, contact the ASH Scientific Affairs Manager, Alice Kuaban, MS, at [email protected].